what to do to deprive tumors from nutrients
There's no shortage of ways to impale a cancer cell. Cut it out, poison it, boom it with radiations, shower it with killer immune cells—they all become the job done. Merely there is a shortage of good ways to kill cancer cells. One that knocks out all the bad cells in ane swipe, leaving the good ones unscathed, and that doesn't allow the disease to return. We accept nevertheless to find that perfect treatment, whether it'due south one miracle drug or several therapies cleverly combined. Until nosotros practise, cancer will continue to kill, and the medicine that stops it will continue to hurt.
For Kivanç Birsoy, the ideal cancer treatment doesn't impale cancer cells with violent attacks at all. He wants to merely stop feeding them and let them die.

Birsoy, the Chapman Perelman Banana Professor, has taken this unproblematic premise—that cancer cells demand nutrients to survive—and built a sophisticated inquiry program around it. His piece of work is driven by a vision of the time to come where patients survive equally their tumors, starved of the nutrients they depend on to grow, wither away. The fundamental to defeating cancer cells, he says, is to understand their metabolism.
Similar many proficient ideas, Birsoy's is not new. The report of cellular metabolism began virtually a century ago and has long been perceived every bit settled science. The textbooks have been written, the Nobels awarded, and the globe has moved on to sexier subjects.
First nosotros figure out what cancer cells need that other cells don't. Then nosotros devise a way to deprive them of information technology.
But the scientists who pioneered the field, who asked the important questions and wrote those textbooks, were limited by their experimental tools, Birsoy explains as he sits in his office in Rockefeller'south newest research building. "With the tools nosotros have now, genetic tools, I tin can go back and ask those questions again," he says, "and get more than sophisticated answers."
When it comes to metabolism, cancer cells are remarkably adjustable. They have several tricks they can employ to maintain their growth, even in the face of inhospitable atmospheric condition that would leave other cells lifeless.
For ane, they can tweak their own metabolic processes, a lethal power that is unique in homo biology—heart cells tin can't do it, encephalon cells can't practice it. Deprived of sufficient blood flow due to a centre set on or stroke, those normal tissues die. But cancer cells are somehow able to crouch down and pull through, and, having survived these hostile conditions, they go on to thrive and multiply.
All the same, in that location are some nutrients even cancer cells tin't live without, which is why they have a second trick up their sleeve: the power to import what they need from the environment instead of producing it themselves. And it is hither that Birsoy sees an opportunity. Working with cells derived from lung, breast, blood, and other types of cancer, his program is to effigy out what cancer cells need that other cells don't, and so devise a mode to deprive them of it.
Cancer cells abound fast. In fact, their ability to grow and divide rapidly, and outpace the cells of healthy tissue, is exactly what makes them so deadly. But fast can hateful sloppy. Although cancerous tissue tin create its own blood vessels, for instance, the new supply is often non enough to meet the cells' need. Despite their varied diet, they detect themselves facing a scarcity of the oxygen and nutrients they demand to survive.
In an experiment, Birsoy subjected 28 cancer cells lines derived from patients to low oxygen weather. None of them were able to synthesize an amino acid called aspartate, which they require to abound. Only six of the 28 overcame this hindrance by altering their metabolism and ingesting aspartate from their environment. And having successfully outsourced aspartate production to their neighbors, these cells continued to grow, separate, and proliferate. Like near of Birsoy'south piece of work, the written report was washed in vivo, using tumor tissue grafted onto mice, a method that provides a more than consummate picture of biological events than experiments conducted in cell civilization.
The findings excite Birsoy for two reasons. First, they provide clear-cut evidence supporting the general hypothesis that cancer cells are able to modify their metabolism to go the nutrients they need to grow. And second, they show the importance of aspartate in particular; tumors can't grow in low oxygen settings without aspartate, which makes limiting its availability a potentially viable cancer therapy.
Such a treatment, he believes, would target cancer cells without affecting nearby healthy tissue.
60-six years ago, a series of studies began that led to a similar discovery. Researchers working with guinea pig serum found that cells with a particular form of cancer, astute lymphoblastic leukemia, are unable to produce an amino acid chosen asparagine. It'south similar to the situation with aspartate, with a key departure: The inability to produce asparagine was due to a rare internal bibelot, not an external factor similar oxygen level.
"There is a small fraction of cancers that cannot brand certain metabolites or nutrients that all other cells are able to make," Birsoy explains. "Then they naturally become dependent on taking it from the exterior." Since the 1960s, oncologists have exploited the leukemia cells' asparagine dependency by treating patients with a drug called L-asparaginase, which depletes all the asparagine in their blood. Equally a result, the survival rate for astute lymphoblastic leukemia, which typically strikes children between ages two and ten, has reached nearly 90 per centum.
Birsoy wondered whether in that location were other claret cancers with the same kind of rare defect—cells that were unable to make necessary nutrients and that could therefore exist targeted by depleting that food. Soon, his grouping plant a rare cancer called ALK+ anaplastic large-cell lymphoma whose cells can't synthesize cholesterol, an essential building cake for membranes. "If you lot deplete cholesterol from the environs," says Birsoy, "these cells dice, even though normal cells don't care." It was the showtime such discovery since 1953.
With the advantage of tools that earlier researchers could non have imagined, including a CRISPR-based genetic screen that targeted 200 enzymes involved in the metabolism of the ALK+ lymphoma cells, Birsoy apace honed in on the culprit. (Amidst other things, CRISPR, a gene-editing system, makes information technology possible to deactivate a specific gene in a cell in order to decide what the cistron does and whether the jail cell can survive without information technology.)
In this instance, when the gene for a specific receptor, LDLR, was knocked out by the CRISPR screen, the cells died because they could not import cholesterol from the extracellular environment. That makes the LDLR pathway what Birsoy calls a targetable liability, one that could be exploited past devising a treatment to prevent the lymphoma cells from taking upwardly cholesterol.
The decades-long gap betwixt the asparagine and cholesterol discoveries, Birsoy says, wasn't for a lack of trying. Postwar scientists in fact spent a great bargain of try hunting for boosted metabolic dependencies, only they were held back by the limitations of their methods and applied science. Then, in the 1980s, the search for cancer genes took center stage and fundamental metabolism inquiry went out of style.
"Because these pathways were in biochemistry textbooks, there was supposedly null left to learn about them."
"I remember people thought they knew everything about it and that it's boring," Birsoy says. "Because these pathways were in biochemistry textbooks, there was supposedly nil left to learn about them."
Now, cellular metabolism is alluring a new generation of scientists who are using 21st-century tools to revivify the field. Birsoy, a native of Turkey, is a Rockefeller alumnus who did his graduate work in Jeffrey M. Friedman's lab, where his focus was obesity. Equally a postdoc at the Whitehead Plant, his interest shifted to cancer. In lodge to study the metabolism of tumor cells, he began to design new tools, including an musical instrument for mimicking the nutrient-deprived environs within tumors.
In that location are many good reasons to larn more near cellular metabolism. For Birsoy, a major 1 is finding new means to adjourn cancer, just he has other applications in mind besides. Every jail cell in the body converts nutrients into energy, and the recipes they use are diverse. If Birsoy'due south work can uncover new details into the workings of, say, fat cells or pancreatic cells, it could lead to a new framework for understanding obesity or diabetes.
Of particular interest to Birsoy are mitochondrial disorders. When he talks about them, you tin hear the mix of fascination, frustration, and resolve that drives so much of his work.
"With mitochondrial disorders," he says, "as with other inborn errors, nosotros know what the trouble is, we know the genetics. Just nosotros don't know how to connect the two."
In some cases, mitochondrial disease makes people deaf; in others it causes seizures; and in still others the result is neuropathy or muscular cloudburst. And despite decades of research, Birsoy says, scientists withal have no inkling why dysfunctions of mitochondria, the cellular organelles that procedure nutrients into energy, cause these disorders. All we know is that somehow a metabolic procedure has gone awry.
Birsoy's work has shown that mitochondria play an of import role in synthesizing aspartate, the same amino acid that cancer cells sometimes steal from their neighbors. When those mitochondria are dysfunctional, he says, their aspartate levels are depression—and supplying the cells with aspartate restores their function. Birsoy suspects that aspartate depletion might exist a root cause of the mitochondrial affliction, and that supplementing aspartate might be an effective treatment strategy. Only it's a theory that remains to be tested.
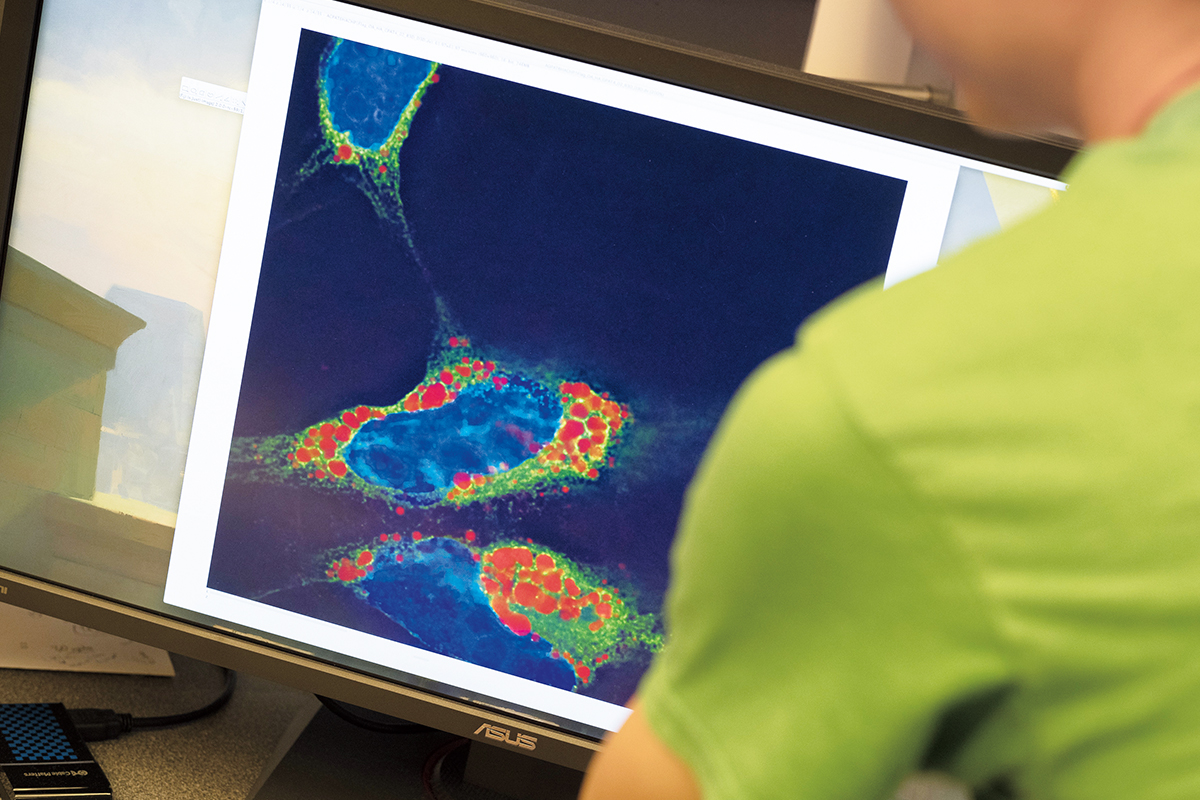
Equally with cellular metabolism, our understanding of how mitochondria function got frozen at some signal in the history of biological discovery, Birsoy says, and it hasn't been revisited. In some sense, nosotros don't even know what mitochondria are. The initial idea was that they were a powerhouse organelle, but that's conspicuously not the full picture, and they probably do different things in different types of cells. "It's time to go back and figure out exactly what the office of mitochondria is in different prison cell types," Birsoy says. "In neurons, what's the function? In muscle, what'due south the office? And in cancer cells, what's the function?"
Metabolism is universal—every jail cell needs nutrients to survive. And although Birsoy'southward work has enormous potential, there'south besides a mountain of his predecessors' crumbling experiments to revisit.
"The style I look at this is, if you lot don't take a cure for something, that means y'all don't know enough nearly it," he says. "We treat cancer and people may alive five weeks, 5 months, or five years longer with existing therapies. Only also often the treatment fails and the patients dice. And that ways there is a lot more to detect."
Regardless of what the textbooks say.
stapletonexciation91.blogspot.com
Source: https://seek.rockefeller.edu/cancers-big-appetite/
0 Response to "what to do to deprive tumors from nutrients"
Post a Comment